

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Gthe following reaction is the first step in the production of nitric acid from ammonia. 4nh3(g) 5o2...
Questions in other subjects:










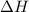
![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0383/2489/18a63.png)

![\Delta H_{rxn}=[(4\times \Delta H_f_{(NO(g))})+(6\times \Delta H_f_{(H_2O(g))})]-[(4\times \Delta H_f_{(NH_3(g))})+(5\times \Delta H_f_{(O_2)})]](/tpl/images/0383/2489/02e37.png)
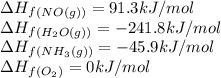
![\Delta H_{rxn}=[(4\times (91.3))+(6\times (-241.8))]-[(4\times (-45.9))+(5\times (0))]\\\\\Delta H_{rxn}=-902kJ](/tpl/images/0383/2489/6f6da.png)


