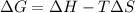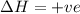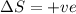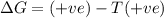Chemistry, 20.11.2019 20:31 Legoman29305
Consider a reaction that has a positive δh and a positive δs. which of the following statements is true? a) this reaction will be spontaneous only at high temperatures. b) this reaction will be spontaneous at all temperatures. c) this reaction will be nonspontaneous at all temperatures. d) this reaction will be nonspontaneous only at high temperatures. e) it is not possible to determine without more information.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2


Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Consider a reaction that has a positive δh and a positive δs. which of the following statements is t...
Questions in other subjects:




English, 06.11.2020 04:40

Social Studies, 06.11.2020 04:40




Mathematics, 06.11.2020 04:40

Mathematics, 06.11.2020 04:40

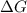 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous