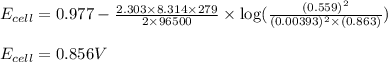Chemistry, 20.11.2019 17:31 hoosierkid5633
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (aq) + 2 h+ + 2 e- → 2 h2o eo = 1.78 v what will the potential of this cell be when [ag+] = 0.559 m, [h+] = 0.00393 m, and [h2o2] = 0.863 m?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, foreignking02
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:00, krystalhurst97
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
You know the right answer?
Agalvanic cell is based on the following half-reactions at 279 k: ag+ + e- → ag eo = 0.803 v h2o2 (...
Questions in other subjects:

Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00



Mathematics, 17.11.2020 22:00


Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00


Mathematics, 17.11.2020 22:00

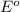 potential will always get reduced.
potential will always get reduced.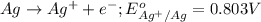 ( × 2)
( × 2)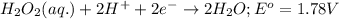

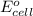 of the reaction, we use the equation:
of the reaction, we use the equation: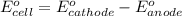
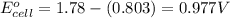
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Ag^{+}]^2}{[H^{+}]^2[H_2O_2]}](/tpl/images/0383/1046/4a00d.png)
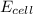 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[Ag^{+}]=0.559M](/tpl/images/0383/1046/b53ec.png)
![[H^{+}]=0.00393M](/tpl/images/0383/1046/74117.png)
![[H_2O_2]=0.863M](/tpl/images/0383/1046/04f5b.png)