
Chemistry, 20.11.2019 01:31 orlando19882000
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the tea is freshly brewed and has an initial temperature of 28.29 °c. you add ice to cool it. if the heat of fusion of ice is 6.020 kj/mol and each ice cube contains exactly 1 mol of water, how many ice cubes are necessary to cool the tea to 0.41 °c? the specific heat of the "tea" is 4.184 j/g*c.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1



Chemistry, 23.06.2019 15:00, WampWamp8751
For part 1, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least copper(ii) nitrate to the well with the most copper(ii) nitrate. which wells had the most distinct precipitate? for part 2, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(ii) sulfate to the well with the most iron(ii) sulfate. which wells had the most distinct precipitate? for part 3, describe the changes in the colors of the well, if any, as you go from well 1 to well 9—that is, as you go from the well with the least iron(iii) nitrate to the well with the most iron(iii) nitrate. which wells had the most distinct precipitate?
Answers: 3
You know the right answer?
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the t...
Questions in other subjects:


Chemistry, 20.09.2020 02:01




Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01


Mathematics, 20.09.2020 02:01

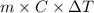
 = change in temperature =
= change in temperature = 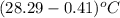 =
= 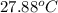
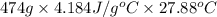
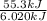
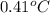 .
.

