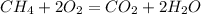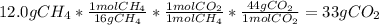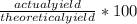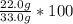Chemistry, 20.11.2019 00:31 Demondevilg
Astudent carried out this reaction with methane as the limiting reagent. a 12.0 g quantity of methane was used, and the student collected 22.0 g of carbon dioxide. what was the percent yield?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 06:30, OsoDeOro7968
Which of these natural resources is non-renewable a. corn b. wind c. geothermal d. natural gas
Answers: 2

Chemistry, 23.06.2019 09:30, noeliaalvarado
Which element below could be and isotope of this atom
Answers: 1
You know the right answer?
Astudent carried out this reaction with methane as the limiting reagent. a 12.0 g quantity of methan...
Questions in other subjects:







Mathematics, 24.04.2021 01:00

Mathematics, 24.04.2021 01:00

History, 24.04.2021 01:00

Chemistry, 24.04.2021 01:00