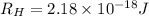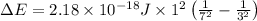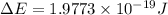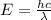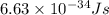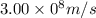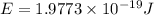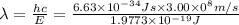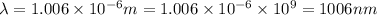Given that rh= 2.18 x 10⁻¹⁸j, 1 nm = 1 x 10⁻⁹m, h = 6.63 x 10⁻³⁴j·s, and c = 3.00 x 10⁸m/s:
c...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:40, raquelqueengucci25
In this synthesis reaction what products will form
Answers: 1

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1


Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Questions in other subjects:

Biology, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00

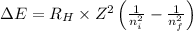
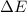 = Energy difference
= Energy difference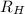 = Rydberg's Constant
= Rydberg's Constant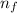 = Final energy level
= Final energy level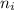 = Initial energy level
= Initial energy level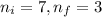 , Z = 1
, Z = 1