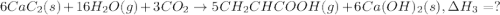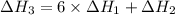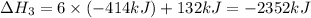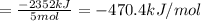Chemistry, 19.11.2019 23:31 sipstick9411
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s) (g) (g) (s) in the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, KindaSmartPersonn
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions in other subjects:

Biology, 11.05.2021 02:50



Mathematics, 11.05.2021 02:50

Mathematics, 11.05.2021 02:50

Health, 11.05.2021 02:50

Advanced Placement (AP), 11.05.2021 02:50



Mathematics, 11.05.2021 02:50

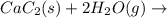
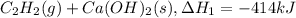 ..[1]
..[1]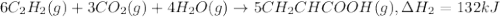 ..[2]
..[2]