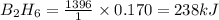Chemistry, 19.11.2019 22:31 ronaldotheexplorer12
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj according to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj a) 1640 kj b) 1430 kj c) 429 kj d) 3070 kj e) 238 kj.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, winstonbendariovvygn
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 a...
Questions in other subjects:

Business, 11.11.2020 21:20



Mathematics, 11.11.2020 21:20




Spanish, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20

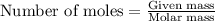 .....(1)
.....(1)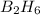 :
: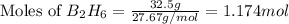
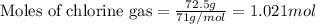
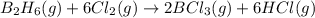
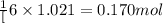 of
of