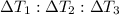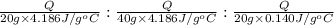Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity is 0.140 joules/gram degree celsius.
water and mercury are put into three identical bowls:
bowl a contains 20 grams of water.
bowl b contains 40 grams of water.
bowl c contains 20 grams of mercury.
the bowls start at the same temperature, and then the same amount of heat is added to each bowl. order the bowls from coolest to warmest, based on their final temperatures.
bowl a

Answers: 2


Other questions on the subject: Chemistry




You know the right answer?
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity...
Questions in other subjects:





Biology, 27.02.2020 04:35



History, 27.02.2020 04:35

Mathematics, 27.02.2020 04:35

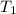
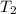
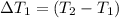
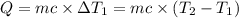
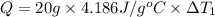
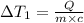
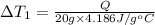 ..[1]
..[1]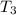
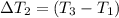
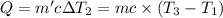
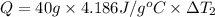
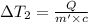
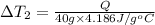 ..[2]
..[2]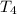
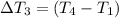
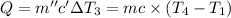
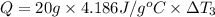
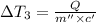
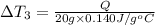 ..[3]
..[3]