

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Consider the complete combustion of glucose (c6h12o6) with o2 and calculate the moles of co2 produce...
Questions in other subjects:

English, 16.12.2020 19:00

History, 16.12.2020 19:00

English, 16.12.2020 19:00


English, 16.12.2020 19:00


English, 16.12.2020 19:00

Mathematics, 16.12.2020 19:00

Chemistry, 16.12.2020 19:00

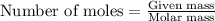
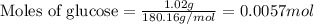
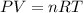
![37^oC=[37+273]K=310K](/tpl/images/0381/5720/20b22.png)
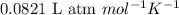
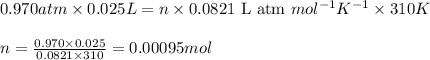
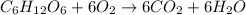
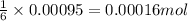 of glucose
of glucose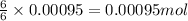 of carbon dioxide
of carbon dioxide

