Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 13:00, nerikzagallegos
Johnny's bakery has 30,900 grams of sugar. a recipe calls for 32 pounds of sugar to be used. how much sugar will be left over? (1 lb=453.59 g).
Answers: 2
You know the right answer?
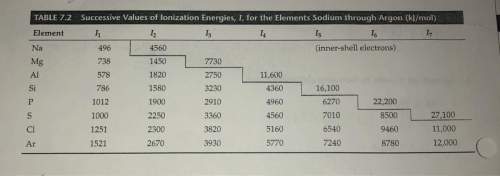
Where would the largest jump in ionization energies be for oxygen? (with the loss of how many elect...
Questions in other subjects:

Mathematics, 25.02.2021 23:10



English, 25.02.2021 23:10


English, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10

Health, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10

Mathematics, 25.02.2021 23:10