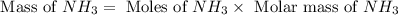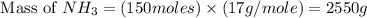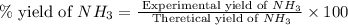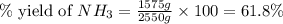Chemistry, 19.11.2019 06:31 jlbradley429
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with nitrogen, 1575 g of ammonia were produced. what is the percent yield of this reaction?
30.8%
61.8%
20.7%
41.5%
more information is needed to solve this problem.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:40, mariobros3090
Numbers with a end value of 4 or lower are rounded up, while with an end value of 5 or higher are rounded down. true or false?
Answers: 1


Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
The reaction n2 + 3 h2 → 2 nh3 is used to produce ammonia. when 450. g of hydrogen was reacted with...
Questions in other subjects:


Mathematics, 08.04.2020 01:17

Mathematics, 08.04.2020 01:17

Mathematics, 08.04.2020 01:17






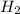 = 450 g
= 450 g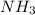 = 17 g/mole
= 17 g/mole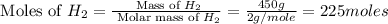
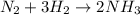
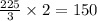 moles of
moles of