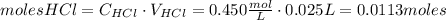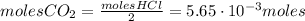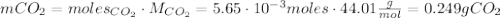Chemistry, 19.11.2019 06:31 caromaybelline71
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the following equation: caco3(s) 2hcl(aq)⟶co2(g) h2o(l) cacl2(aq) a typical antacid contains caco3. if such an antacid is added to 25.0 ml of a solution that is 0.450 m in hcl, how many grams of co2 gas are produced?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Calcium carbonate (caco3) reacts with stomach acid (hcl, hydrochloric acid) according to the followi...
Questions in other subjects:


English, 10.10.2019 02:00



Mathematics, 10.10.2019 02:00





Mathematics, 10.10.2019 02:00