
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. chlorine trifluoride is prepared by the reaction cl2 (g) 3f2 (g) ⟶ 2clf3 (g). write the equation that relates the rate expressions for this reaction in terms of the disappearance of cl2 and f2 and the formation of clf3.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile co...
Questions in other subjects:



Arts, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00


Mathematics, 10.03.2021 22:00



Spanish, 10.03.2021 22:00

Social Studies, 10.03.2021 22:00

![Rate=-\frac{d[Cl_2]}{dt}=-\frac{1}{3}\frac{d[F_2]}{dt}=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/d2692.png)
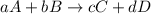
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0380/6825/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0380/6825/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0380/6825/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0380/6825/d4b94.png)
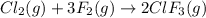
![\text{Rate of disappearance of }Cl_2=-\frac{d[Cl_2]}{dt}](/tpl/images/0380/6825/4403e.png)
![\text{Rate of disappearance of }F_2=-\frac{1}{3}\frac{d[F_2]}{dt}](/tpl/images/0380/6825/c24a1.png)
![\text{Rate of formation of }ClF_3=+\frac{1}{2}\frac{d[ClF_3]}{dt}](/tpl/images/0380/6825/1400a.png)


