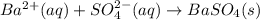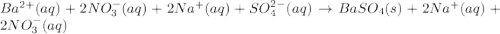Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined. upon mixing, a thick, milky white precipitate formed. after centrifugation, the solution above the precipitate was found to be clear and colorless. based on these observations, determine if a reaction occurred. if so, write the balanced chemical equation and net ionic equation for the reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, browndalton55
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
Approximately 1 ml of two clear, colorless solutions-0.1 m ba(no3)2 and 0.1 m na2so4- were combined....
Questions in other subjects:

Mathematics, 03.12.2019 23:31

Mathematics, 03.12.2019 23:31

English, 03.12.2019 23:31

History, 03.12.2019 23:31

Mathematics, 03.12.2019 23:31


Mathematics, 03.12.2019 23:31

Computers and Technology, 03.12.2019 23:31


Mathematics, 03.12.2019 23:31