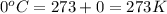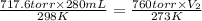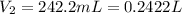Chemistry, 19.11.2019 03:31 carapiasebas
Atrial of this decomposition experiment, using different quantities of reactants than those listed in the question above produced the following data: volume of o2 produced at room conditions 280 ml barometric pressure 740 torr temperature of water 24°c termperature of o2 25°c vapor pressure due to water at 25°c 22.4 torr for the conditions listed above, calculate the volume of o2(g) produced at standard conditions of temperature and pressure. (enter your answer in liters)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3


Chemistry, 23.06.2019 08:00, oopsorry
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
Atrial of this decomposition experiment, using different quantities of reactants than those listed i...
Questions in other subjects:

Mathematics, 04.02.2021 20:30

Mathematics, 04.02.2021 20:30

Mathematics, 04.02.2021 20:30


Mathematics, 04.02.2021 20:30




Mathematics, 04.02.2021 20:30

 produced at standard conditions of temperature and pressure is 0.2422 L
produced at standard conditions of temperature and pressure is 0.2422 L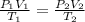
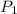 = initial pressure of
= initial pressure of 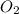 gas = (740-22.4) torr = 717.6 torr
gas = (740-22.4) torr = 717.6 torr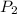 = final pressure of
= final pressure of 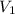 = initial volume of
= initial volume of 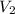 = final volume of
= final volume of 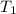 = initial temperature of
= initial temperature of 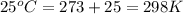
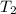 = final temperature of
= final temperature of