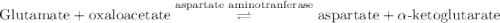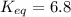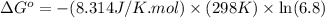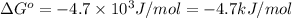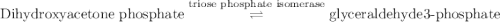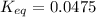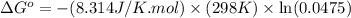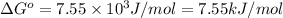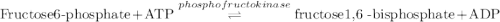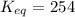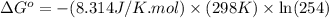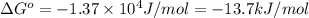Chemistry, 19.11.2019 03:31 joseenrique02
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each of the following metabolically important enzyme-catalyzed reactions, using the equilibrium constants given for the reactions at 25 °c and ph 7.0. ( a ) glutamate + oxaloacetate aspartate aminotranferase ⇌ aspartate + α -ketoglutarate k ′ eq = 6.8 ( b ) dihydroxyacetone phosphate triose phosphate isomerase ⇌ glyceraldehyde 3 -phosphate k ′ eq = 0.0475 ( c ) fructose 6 -phosphate + atp phosphofructokinase ⇌ fructose 1 , 6 -bisphosphate + adp k ′ eq = 254

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Calculation of δg′° from an equilibrium constant calculate the standard free-energy change for each...
Questions in other subjects:



Computers and Technology, 23.08.2019 03:10

Computers and Technology, 23.08.2019 03:10




Computers and Technology, 23.08.2019 03:10

Engineering, 23.08.2019 03:10

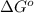 for the reaction is -4.7 kJ/mol
for the reaction is -4.7 kJ/mol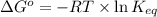
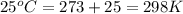
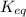 = equilibrium constant
= equilibrium constant