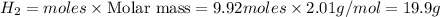Chemistry, 19.11.2019 02:31 kaylallangari2145
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are heated together. if 20.0 g of hydrogen and 20.0 g of iodine are heated, forming 10.0 g of hydrogen iodide, what mass of hydrogen remains unreacted? a. 10.0 g hydrogen remains b. 10.9 g hydrogen remains c. 15.0 g hydrogen remains d. 19.9 g hydrogen remains.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


You know the right answer?
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are h...
Questions in other subjects:

English, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Biology, 14.09.2020 14:01

Social Studies, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

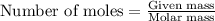
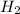
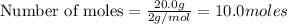
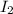
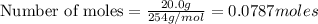
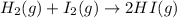
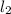 require=
require=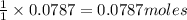 of
of