Balance the following equation:
k2cro4+na2so3+hcl? kcl+na2so4+crcl3+h2o
gen...

Chemistry, 19.11.2019 02:31 sheyenne143
Balance the following equation:
k2cro4+na2so3+hcl? kcl+na2so4+crcl3+h2o
generally coefficients of 1 are omitted from balanced chemical equations. when entering your answer, include coefficients of 1 as required for grading purposes.
enter the coefficients for each compound, separated by commas, in the order in which they appear in the equation (e. g., 1,2,3,4,5,6,7).
part b
in the process of oxidizing i? to i2, so42? is reduced to so2. how many moles of so2 are produced in the formation of one mole of i2?
express your answer numerically in moles.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:31, woodfordmaliky
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1

Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
Questions in other subjects:





English, 17.12.2020 02:10

Mathematics, 17.12.2020 02:10



Mathematics, 17.12.2020 02:10

English, 17.12.2020 02:10

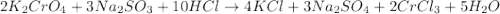
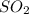 is produced.
is produced.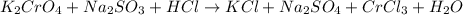
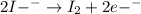
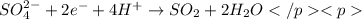
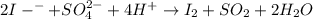
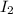 is produced, 1 mole of
is produced, 1 mole of 

