
Chemistry, 19.11.2019 02:31 AllyJungkookie
Aspirin (c9h8o4, molecular mass 180.2 g/mol) is synthesized by the reaction of salicylic acid (c7h6o3, molecular mass 138.1 g/mol) with acetic anhydride (c4h6o3, molecular mass 102.1 g/mol) according to the reaction 2 c7h6o3 (s) + c4h6o3(l) → 2c9h8o4(s)+ h2o(l) 2.0g of salicylic acid and 5.4 g of acetic anhydride are mixed and the reaction is allowed to go to completion. do each of the following: a. identify the limiting reagent. b. state how many grams of the excess reagent remain. c. state how many grams of product are produced.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Aspirin (c9h8o4, molecular mass 180.2 g/mol) is synthesized by the reaction of salicylic acid (c7h6o...
Questions in other subjects:

Physics, 17.07.2019 09:30






Arts, 17.07.2019 09:30


Mathematics, 17.07.2019 09:30

Mathematics, 17.07.2019 09:30

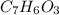 is the limiting reagent.
is the limiting reagent.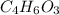 remain.
remain. are produced.
are produced.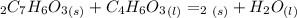
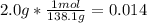
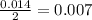
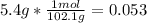
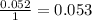
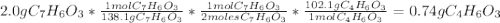 reacts
reacts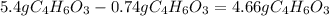 remain
remain are produced.
are produced.


