Chemistry, 19.11.2019 01:31 fluffylove83
Achemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. a. calculate the volume of solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Achemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilut...
Questions in other subjects:


Mathematics, 21.01.2021 20:00

French, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00


Chemistry, 21.01.2021 20:00


Mathematics, 21.01.2021 20:00

History, 21.01.2021 20:00

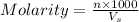
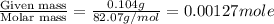
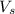 = volume of solution in ml
= volume of solution in ml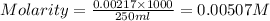
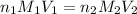
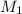 = molarity of
= molarity of 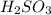 solution = 0.00507 M
solution = 0.00507 M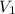 = volume of
= volume of 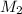 = molarity of
= molarity of 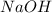 solution = 0.0700 M
solution = 0.0700 M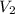 = volume of
= volume of 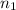 = valency of
= valency of 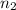 = valency of
= valency of