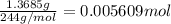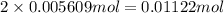Chemistry, 19.11.2019 01:31 sixtomomtermont
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the mixture weighed 72.248g. when empty the beaker weighed 69.748g. the mixture was determined to contain 45.26% sand.
(a) what is the % and mass of the hydrate in the mixture?
(b) if the mixture was selectively decomposed by heating, how many grams and moles of water would be lost?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, S4NCHEZ28
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1



Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the m...
Questions in other subjects:


Mathematics, 05.09.2021 02:00

Mathematics, 05.09.2021 02:00



Mathematics, 05.09.2021 02:00


Mathematics, 05.09.2021 02:00

English, 05.09.2021 02:00