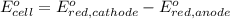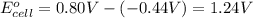Chemistry, 19.11.2019 01:31 burnsmykala23
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given that the standard reduction potential of ag+ to ag (s) is +0.80 v and the standard reduction potential of fe2+ to fe (s) is −0.44 v, calculate the standard cell potential, e°cell.−1.24 v1.24 v2.04 v0.36 v

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Avoltaic cell uses the following reaction: 2ag+ (aq, 1 m) + fe (s) ↔ 2ag (s) + fe2+ (aq, 1 m) given...
Questions in other subjects:

Mathematics, 22.09.2020 08:01



Social Studies, 22.09.2020 08:01


History, 22.09.2020 08:01


Mathematics, 22.09.2020 08:01

Mathematics, 22.09.2020 08:01

Biology, 22.09.2020 08:01

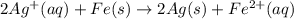
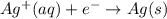
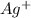 to Ag=
to Ag=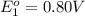
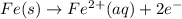
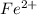 to Fe=
to Fe=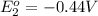
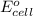 of the reaction, we use the equation:
of the reaction, we use the equation: