Chemistry, 19.11.2019 00:31 keyonaemanieevans
Manganese reacts with hydrochloric acid to produce manganese(ii) chloride and hydrogen gas. mn(s)+2hcl(aq) ? mncl2(aq)+h2(g)
part a when 0.650g mn is combined with enough hydrochloric acid to make 100.0 ml of solution in a coffee-cup calorimeter, all of the mn reacts, raising the temperature of the solution from 23.0? c to 28.2? c. find ? hrxn for the reaction as written. (assume that the specific heat capacity of the solution is 4.18 j/g? c and the density is 1.00 g/ml.) express your answer using three significant figures. ? hrxn = kj

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2


You know the right answer?
Manganese reacts with hydrochloric acid to produce manganese(ii) chloride and hydrogen gas. mn(s)+2h...
Questions in other subjects:


Mathematics, 12.10.2020 09:01

English, 12.10.2020 09:01

Mathematics, 12.10.2020 09:01

Mathematics, 12.10.2020 09:01



Mathematics, 12.10.2020 09:01


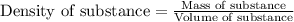
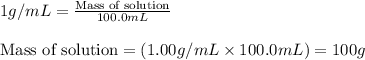
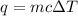
 = change in temperature =
= change in temperature = 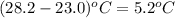
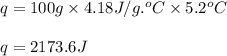
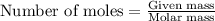
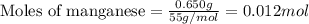
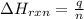
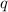 = amount of heat absorbed = 2173.6 J = 2.174 kJ (Conversion used: 1 kJ = 1000 J)
= amount of heat absorbed = 2173.6 J = 2.174 kJ (Conversion used: 1 kJ = 1000 J)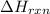 = enthalpy change of the reaction
= enthalpy change of the reaction