
Nh4+ (aq) + no2- (aq) → n2 (g) + h2o (l) experiment [nh4+]i [no2-]i initial rate (m/s) 1 0.24 0.10 7.2 x 10-4 2 0.12 0.10 3.6 x 10-4 3 0.12 0.15 5.4 x 10-4 4 0.12 0.12 4.3 x 10-4 first determine the rate law and rate constant. under the same initial conditions as in experiment 4, calculate [nh4+] at 274 seconds after the start of the reaction. in this experiment, both reactants are present at the same initial concentration. the units should be m, and should be calculated to three significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1



Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Nh4+ (aq) + no2- (aq) → n2 (g) + h2o (l) experiment [nh4+]i [no2-]i initial rate (m/s) 1 0.24 0.10 7...
Questions in other subjects:

Mathematics, 28.01.2020 09:31


Mathematics, 28.01.2020 09:31




Chemistry, 28.01.2020 09:31



English, 28.01.2020 09:31

![\text{Rate}=k[NH_4^+][NO_2^-]](/tpl/images/0380/3184/ed258.png)
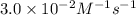
![[NH_4^+]](/tpl/images/0380/3184/5c46c.png) at 274 seconds after the start of the reaction is 0.0604 M
at 274 seconds after the start of the reaction is 0.0604 M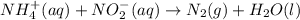
![\text{Rate}=k[NH_4^+]^a[NO_2^-]^b](/tpl/images/0380/3184/00132.png)
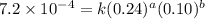 ....(1)
....(1)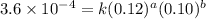 ....(2)
....(2)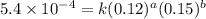 ....(3)
....(3)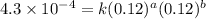 ....(4)
....(4)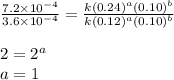
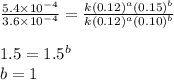
![\text{Rate}=k[NH_4^+]^1[NO_2^-]^1](/tpl/images/0380/3184/43151.png)
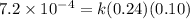
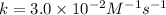
![[NO_2^-]](/tpl/images/0380/3184/10a69.png) are same and the reaction is 1st order for both.
are same and the reaction is 1st order for both.![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0380/3184/ccade.png)
![[A_t]](/tpl/images/0380/3184/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0380/3184/dc622.png) = initial concentration = 0.12 M
= initial concentration = 0.12 M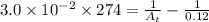
![[A_t]=0.0604M](/tpl/images/0380/3184/69b49.png)


