
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements describes the behavior of the system if pocl is added to the container? (a) the forward reaction will proceed to establish equilibrium.(b) the reverse reaction will proceed to establish equilibrium.(c) the partial pressures of pocl3 and pocl will remain steady while the partial pressure of chlorine increases.(d) the partial pressure of chlorine remains steady while the partial pressures of pocl3 and pocl increase.(e) the partial pressure of chlorine will increase while the partial pressure of pocl decreases.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements...
Questions in other subjects:



Mathematics, 20.07.2020 01:01



Mathematics, 20.07.2020 01:01




Mathematics, 20.07.2020 01:01

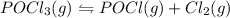
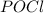 is added to the system
is added to the system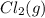 decreases and the partial pressure of
decreases and the partial pressure of 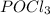 increases.
increases.

