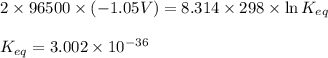What is the value of the equilibrium constant at 25 oc for the reaction between the pair: ag(s) and ni2+(aq) to give ni(s) and ag+(aq) use the reduction potential values for ag+(aq) of +0.80 v and for ni2+(aq) of -0.25 v give your answer using e-notation with no decimal places (e. g., 2 x 10-2 would be 2e-2; and 2.12 x 10-2 would also be 2e- do not include spaces, units, punctuation or anything else silly!

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1


You know the right answer?
What is the value of the equilibrium constant at 25 oc for the reaction between the pair: ag(s) and...
Questions in other subjects:

History, 25.02.2020 22:58


History, 25.02.2020 22:58

Social Studies, 25.02.2020 22:58





History, 25.02.2020 22:58

Mathematics, 25.02.2020 22:58

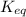 of the reaction is
of the reaction is  .
.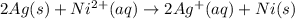
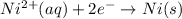
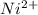 to Ni=
to Ni=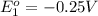
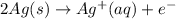
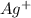 to Ag=
to Ag=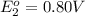
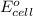 of the reaction, we use the equation:
of the reaction, we use the equation: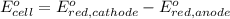
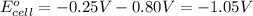

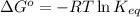
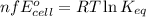
![25^oC=[273+25]=298K](/tpl/images/0380/0449/6a9f9.png)