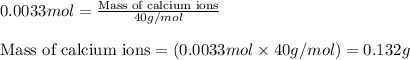Chemistry, 18.11.2019 22:31 kgonzalez200061
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxalate to a sample and measuring the mass of calcium oxalate precipitated. what is the mass percent of calcium in milk if 0.429 g of calcium oxalate, cac2o4, forms in a 125-g sample of milk when excess aqueous sodium oxalate is added? na2c2o4 (aq) + ca2+ (aq) → cac2o4 (s) + 2 na+ (aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
You know the right answer?
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxala...
Questions in other subjects:

Mathematics, 03.07.2019 09:00


Mathematics, 03.07.2019 09:00



Mathematics, 03.07.2019 09:00

Mathematics, 03.07.2019 09:00


History, 03.07.2019 09:00

English, 03.07.2019 09:00

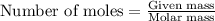 .....(1)
.....(1)
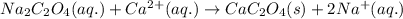
 of calcium ions
of calcium ions