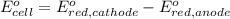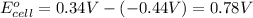In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are cu2+(aq)+2e−→cu(s) and fe(s)→fe2+(aq)+2e− the net reaction is cu2+(aq)+fe(s)→cu(s)+fe2+(aq) use the given standard reduction potentials in your calculation as appropriate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 23.06.2019 10:30, fatheadd2007
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions in other subjects:



Mathematics, 20.04.2020 23:55


English, 20.04.2020 23:55



Mathematics, 20.04.2020 23:55

Spanish, 20.04.2020 23:55

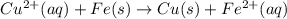
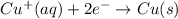
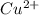 to Cu=
to Cu=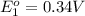
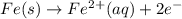
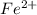 to Fe=
to Fe=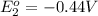
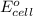 of the reaction, we use the equation:
of the reaction, we use the equation: