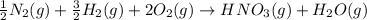Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) 2nh3(g) ah=-92. kj in the second step, ammonia and oxygen react to form nitric acid and water: nh3(9) + 2o2(g) → hno3(9) + h2o(g) ah=-330. kj calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest kj. пkj 1 x ś ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3


Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen...
Questions in other subjects:

Mathematics, 08.01.2021 20:00

World Languages, 08.01.2021 20:00

Computers and Technology, 08.01.2021 20:00


Social Studies, 08.01.2021 20:00


Mathematics, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

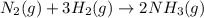 ,
, 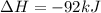
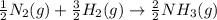 ,
, 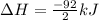
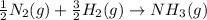 ,
, 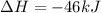 ............ (1)
............ (1)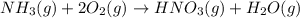
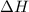 = -330 kJ ............ (2)
= -330 kJ ............ (2)