Chemistry, 18.11.2019 20:31 PlaneGamer5678
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 387mmhg .identify the limiting reactant and determine the theoretical yield of methanol in grams.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3


Chemistry, 23.06.2019 19:00, ciarrap552
The mass of a substance that has a density of 10.0g/ml and a volume of 6.0ml is
Answers: 1

Chemistry, 23.06.2019 23:40, laura52677
If 3.50 g of the unknown compound contained 0.117 mol of c and 0.233 mol of h, how many moles of oxygen, o, were in the sample? express your answer to three significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reactio...
Questions in other subjects:


Social Studies, 19.12.2019 12:31

Computers and Technology, 19.12.2019 12:31



Mathematics, 19.12.2019 12:31

English, 19.12.2019 12:31



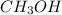 is 0.422 grams.
is 0.422 grams.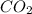 and
and 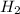 gas.
gas.
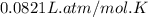
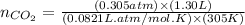
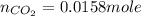

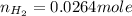
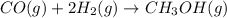
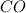
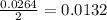 moles of
moles of