
Chemistry, 18.11.2019 20:31 Thunderalesis7855
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solution. first the student prepares a set of cocl2(aq) solutions of known concentration. then the student uses a spectrophotometer to determine the absorbance of each of the standard solutions at a wavelength of 510nm and constructs a standard curve. finally, the student determines the absorbance of the sample of unknown concentration. a wavelength of 510nm corresponds to an approximate frequency of 6×1014s−1. what is the approximate energy of one photon of this light? 9×1047j.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

You know the right answer?
Astudent uses visible spectrophotometry to determine the concentration of cocl2(aq) in a sample solu...
Questions in other subjects:

English, 22.12.2020 07:10

Mathematics, 22.12.2020 07:10

Social Studies, 22.12.2020 07:10


Mathematics, 22.12.2020 07:10





History, 22.12.2020 07:10

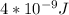
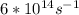
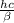 ---- ( 1 )
---- ( 1 )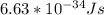
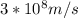
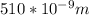
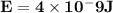
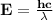
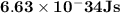
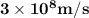
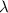 - wavelength = 510nm
- wavelength = 510nm

