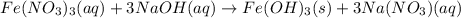Chemistry, 18.11.2019 19:31 meghan0123
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably when a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably a. iron (iii) nitrate. b. sodium hydroxide. c. iron (iii) hydroxide. d. sodium nitrate. e. none of the above

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored pr...
Questions in other subjects:

Mathematics, 10.11.2020 22:10



Computers and Technology, 10.11.2020 22:10

Mathematics, 10.11.2020 22:10

History, 10.11.2020 22:10


Biology, 10.11.2020 22:10