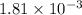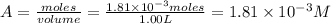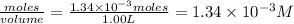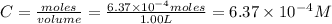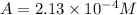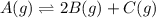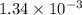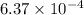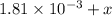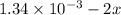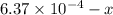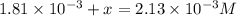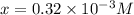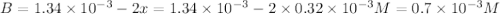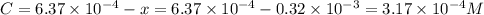Be sure to answer all parts. compound a decomposes according to the equation a(g) ⇌ 2 b(g) + c (g) a sealed 1.00−l container initially contains 1.81 × 10−3 mol of a(g), 1.34 × 10−3 mol of b(g), and 6.37 × 10−4 mol of c(g) at 100°c. at equilibrium, [a] is 2.13 × 10−3 m. find [b] and [c]. solve for the equilibrium concentrations of b and c. [b]eq × 10 m [c]eq × 10 m

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Be sure to answer all parts. compound a decomposes according to the equation a(g) ⇌ 2 b(g) + c (g) a...
Questions in other subjects:

English, 17.02.2022 14:40



English, 17.02.2022 14:40




Geography, 17.02.2022 14:40

English, 17.02.2022 14:40

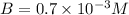
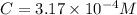
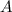 =
=