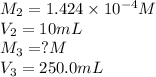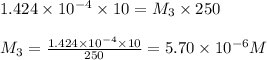Chemistry, 18.11.2019 18:31 sandeebassett3
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions instead of measuring a very small mass. a solution was prepared by dissolving 0.360 g of kno3 in enough water to make 500. ml of solution. a 10.0 ml sample of this solution was transferred to a 500.0-ml volumetric flask and diluted to the mark with water. then 10.0 ml of the diluted solution was transferred to a 250.0-ml flask and diluted to the mark with water. what is the final concentration of the kno3 solution? 7.91 × 10-9 m1.42 × 10-4 m5.70 × 10-6 m2.85 × 10-6 m7.12 × 10-3 m

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions...
Questions in other subjects:

Physics, 09.12.2019 23:31


Social Studies, 09.12.2019 23:31

English, 09.12.2019 23:31

Mathematics, 09.12.2019 23:31


Mathematics, 09.12.2019 23:31


English, 09.12.2019 23:31

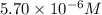
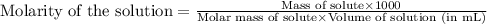
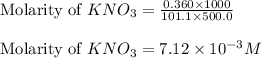
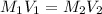 .......(1)
.......(1)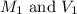 are the molarity and volume of the concentrated
are the molarity and volume of the concentrated 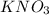 solution
solution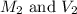 are the molarity and volume of diluted
are the molarity and volume of diluted 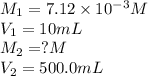
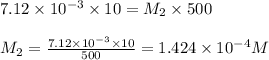
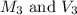 are the molarity and volume of diluted
are the molarity and volume of diluted