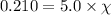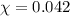Chemistry, 16.11.2019 07:31 Tyasiapalair371
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the surface). what mole fraction of oxygen is necessary in order for the partial pressure of oxygen in the gas mixture the diver breathes to be 0.210 atm?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Assume a scuba diver is working at a depth where the total pressure is 5.0 atm (about 50 m below the...
Questions in other subjects:

Mathematics, 05.05.2021 08:40




Mathematics, 05.05.2021 08:40

Chemistry, 05.05.2021 08:40

Chemistry, 05.05.2021 08:40

Mathematics, 05.05.2021 08:40


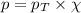
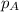 = partial pressure of oxygen = 0.210 atm
= partial pressure of oxygen = 0.210 atm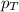 = total pressure = 5.0 atm
= total pressure = 5.0 atm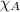 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?