
Chemistry, 16.11.2019 05:31 isabelle1670
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj if 60.0 ml of 0.200 m h3po4 is mixed with 60.0 ml of 0.600 m koh initially at 23.43 °c, predict the final temperature of the solution, assuming its density is 1.13 g/ml and its specific heat is 3.78 j/(g·°c). assume that the total volume is the sum of the individual volumes.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, britotellerialuis
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj i...
Questions in other subjects:


Mathematics, 01.04.2021 19:40




Mathematics, 01.04.2021 19:40

Spanish, 01.04.2021 19:40


Mathematics, 01.04.2021 19:40

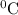
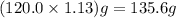
 =
= 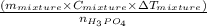
 represents change in temperature and n is number of moles
represents change in temperature and n is number of moles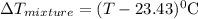
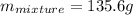 and
and 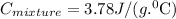
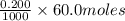 = 0.012 moles
= 0.012 moles![(173.2\times 10^{3})J=\frac{[(135.6g)\times (3.78J.g^{-1}.^{0}\textrm{C}^{-1})\times (T-23.43)^{0}\textrm{C}]}{0.012}](/tpl/images/0377/0998/64167.png)


