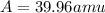Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and the abundances of each of the isotopes: argon36 (35.97 amu; 0.337%), argon-38 (37.96 amu; 0.063%), argon-40 (39.96 amu; 99.600%). 1. 119.89 amu 2. 39.95 amu 3. 39.96 amu 4. 35.97 amu 5. none of these 6. 37.96 amu 7. 37.95 amu 8. 35.96 amu

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Cooldude3966
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic ma...
Questions in other subjects:


Biology, 18.01.2021 08:10

Chemistry, 18.01.2021 08:10

Mathematics, 18.01.2021 08:10

Mathematics, 18.01.2021 08:10

History, 18.01.2021 08:10

Mathematics, 18.01.2021 08:10

Mathematics, 18.01.2021 08:10


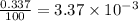
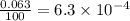
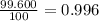
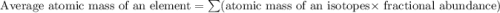
![A=\sum[(35.97\times 3.37\times 10^{-3})+(37.96\times 6.3\times 10^{-4})+(39.96\times 0.996)]](/tpl/images/0377/0178/15521.png)