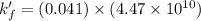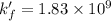A110.0 ml sample of 0.040 m ca2+ is titrated with 0.040 m edta at ph 9.00. the value of logkf for the ca2+−edta complex is 10.65 and the fraction of free edta in the y4− form, − , is 0.041 at ph 9.00. what is k′f , the conditional formation constant, for ca2+ at ph 9.00?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
A110.0 ml sample of 0.040 m ca2+ is titrated with 0.040 m edta at ph 9.00. the value of logkf for th...
Questions in other subjects:


Mathematics, 12.05.2021 04:10

Computers and Technology, 12.05.2021 04:10

Social Studies, 12.05.2021 04:10






Mathematics, 12.05.2021 04:10



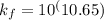
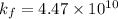
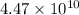
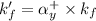
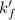 = conditional formation constant = ?
= conditional formation constant = ? = activity coefficient at pH 9.00 = 0.041
= activity coefficient at pH 9.00 = 0.041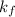 = formation constant =
= formation constant =