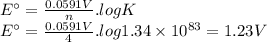The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is given below. 2 h2(g) + o2(g) → 2 h2o(l) k = 1.34 ✕ 1083 (a) calculate ℰ° and δg° at 298 k for the fuel-cell reaction. ℰ° v δg° kj (b) predict the signs of δh° and δs° for the fuel-cell reaction. δh°: positive negative δs°: positive negative (c) as temperature increases, does the maximum amount of work obtained from the fuel-cell reaction increase, decrease, or remain the same? explain. since δs is , as t increases, δg becomes more . therefore, the maximum work obtained will as t increases.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
The overall reaction and equilibrium constant value for a hydrogen-oxygen fuel cell at 298 k is give...
Questions in other subjects:


English, 23.01.2020 21:31



Mathematics, 23.01.2020 21:31





Chemistry, 23.01.2020 21:31