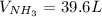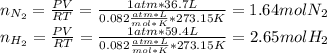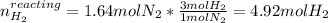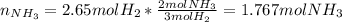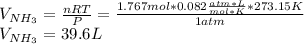Chemistry, 16.11.2019 03:31 kedjenpierrelouis
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh3(g). what volume of ammonia (in l) could be produced by the reaction of 59.4 liters of hydrogen with 36.7 liters of nitrogen at a constant pressure and temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
You know the right answer?
Consider the reaction between hydrogen gas and nitrogen gas to form ammonia: 3 h2(g) + n2(g) → 2 nh...
Questions in other subjects:

History, 22.10.2020 01:01

Arts, 22.10.2020 01:01



English, 22.10.2020 01:01


Physics, 22.10.2020 01:01


English, 22.10.2020 01:01