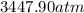Chemistry, 16.11.2019 03:31 prettyluhangel
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by the van der waals equation? the ideal gas law constant is 0.08206 [l•atm] / [mol•k]. for co2, the pressure correction constant is 3.658 l2•atm / mol 2, and the volume correction constant is 0.04286 l / mol.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Consider 20.0 moles of co2 in a 1.0 liter container at 300.0 k. what is the pressure predicted by th...
Questions in other subjects:





History, 04.03.2021 22:40

Mathematics, 04.03.2021 22:40

Biology, 04.03.2021 22:40


Mathematics, 04.03.2021 22:40

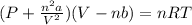
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}][1.0L-(20.0mol\times0.04286 L.mol^{-1} )]=(20.0mol)\times (0.08206L.atm.mol^{-1}.K^{-1})\times (300.0K)](/tpl/images/0376/9858/97073.png)
![[P+\frac{(20.0mol)^{2}\times (3.658L^{2}.atm.mol^{-2})}{(1.0L)^{2}}]](/tpl/images/0376/9858/353d5.png) =
=