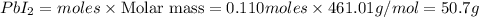Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipitate. 2 k i ( a q ) + p b ( n o 3 ) 2 ( a q ) ⟶ p b i 2 ( s ) + 2 k n o 3 ( a q ) 2ki(aq)+pb(nox3)x2(aq)⟶pbix2(s)+2kn ox3(aq) what mass of p b i 2 pbix2 can be formed by adding 0.528 l of a 0.417 m solution of k i ki to a solution of excess p b ( n o 3 ) 2 pb(nox3)x2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipit...
Questions in other subjects:










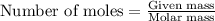
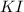
![\text{Number of moles}=molarity\times {\text {Volume in L]}=0.417M\times 0.528L=0.220moles](/tpl/images/0376/8666/592a1.png)
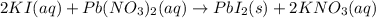
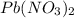 is in excess.
is in excess.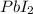
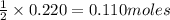 of
of