
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constant kp has a value of 0.003691 based on a reference-state pressure of 1 atm (101,325 pa). derive an algebraic ex- pression for the forward rate coefficient kf . evaluate your expression for a temperature of 1500 k. give units.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, browndalton55
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constan...
Questions in other subjects:



Mathematics, 08.03.2021 17:40




Mathematics, 08.03.2021 17:40



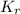 =
= 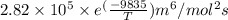
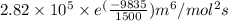
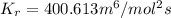
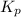 and
and 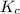 is as follows.
is as follows.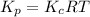
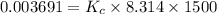
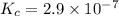
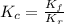
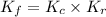
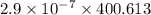
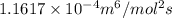
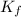 is
is 

