
Chemistry, 16.11.2019 00:31 samueltaye
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric pressure. b. two moles of ammonia gas are cooled from 325 ? °c to 300 °c at 1.2 atm. c. h2o(l) => h2o(g) at 100 °c at atmospheric pressure. d. caco3(s) => cao(s) + co2(g) at 800 °c at atmospheric pressure. e. co2(s) => co2(g) at atmospheric pressure.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
In which one of the following processes is δh = δe? a. 2hi(g) => h2(g) + i2(g) at atmospheric p...
Questions in other subjects:

Biology, 08.04.2020 00:54

Mathematics, 08.04.2020 00:54




Mathematics, 08.04.2020 00:54

History, 08.04.2020 00:54



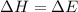
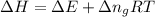
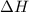 = change in enthalpy
= change in enthalpy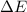 = change in internal energy
= change in internal energy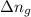 = change in moles
= change in moles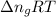 will be zero.
will be zero.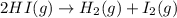 at atmospheric pressure.
at atmospheric pressure.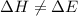
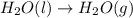 at 100 °C at atmospheric pressure.
at 100 °C at atmospheric pressure.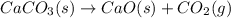 at 800 °C at atmospheric pressure.
at 800 °C at atmospheric pressure.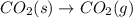 at atmospheric pressure.
at atmospheric pressure.

