
Chemistry, 15.11.2019 20:31 zachinartos1
Substances a, b, and c can all act as oxidizing agents. in solution, a is green, b is yellow, and c is red. in the reactions in which they participate, they are reduced to a-, b-, and c- ions, all of which are colorless. when a solution of c is mixed with one containing b- ions, the color changes from red to yellow. which species is oxidized? which is reduced? when a solution of c is mixed with one containing a- ions, the color remains red. is c a better oxidizing agent than a? is c a better oxidizing agent than b?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Substances a, b, and c can all act as oxidizing agents. in solution, a is green, b is yellow, and c...
Questions in other subjects:







Mathematics, 15.07.2019 13:30

Mathematics, 15.07.2019 13:30





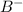 ions changes from red to yellow:
ions changes from red to yellow: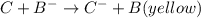
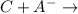 no reaction
no reaction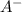 ions which indicates that C was unable to oxidize
ions which indicates that C was unable to oxidize 

