Which of the following are isoelectronic?
a. k⁺¹, na⁺¹, he
b. mg⁺², o⁻², kr
c. n⁻...


Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Questions in other subjects:


English, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Arts, 16.12.2020 18:40


Mathematics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

Mathematics, 16.12.2020 18:40

![[N]=1s^22s^22p^3](/tpl/images/0376/2884/27394.png)
![[N]^{3-}=1s^22s^22p^6](/tpl/images/0376/2884/17dd5.png)
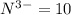
![[O]=1s^22s^22p^4](/tpl/images/0376/2884/336ff.png)
![[O]^{2-}=1s^22s^22p^6](/tpl/images/0376/2884/cd710.png)
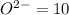
![[Ne]=1s^22s^22p^6](/tpl/images/0376/2884/5b2f7.png)
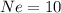
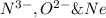 are isoelectronic.
are isoelectronic.


