Chemistry, 15.11.2019 20:31 Mrblunt5613
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g) δh o rxn = −1255.8 kj given δh o f of co2(g) = −393.5 kj/mol and δh o f of h2o(g) = −241.8 kj/mol, find δh o f of c2h2(g).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

You know the right answer?
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g)...
Questions in other subjects:

Mathematics, 02.09.2019 16:30

Chemistry, 02.09.2019 16:30

History, 02.09.2019 16:30



Computers and Technology, 02.09.2019 16:30


Chemistry, 02.09.2019 16:30


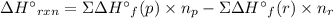
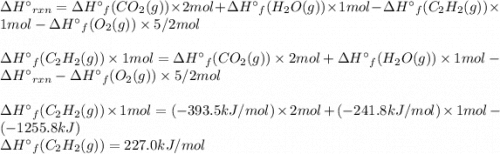
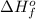 , of C₂H₂ is 227 kJ/mol
, of C₂H₂ is 227 kJ/mol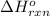 = −1255.8 kJ
= −1255.8 kJ