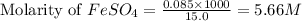In an oxidation-reduction reaction, it required 25.6 ml of a 0.65 m potassium permanganate solution to reach the equivalence point with 15.0 ml of an iron(ii) sulfate solution. what is the molar concentration of the iron(ii) sulfate solution? the net ionic equation for the reaction is:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 02:30, ineedhelp2285
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 06:30, lwattsstudent
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1
You know the right answer?
In an oxidation-reduction reaction, it required 25.6 ml of a 0.65 m potassium permanganate solution...
Questions in other subjects:

Mathematics, 26.03.2020 02:00




Mathematics, 26.03.2020 02:00




History, 26.03.2020 02:00

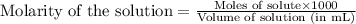 .....(1)
.....(1)
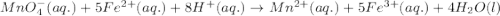
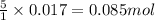 of iron (II) ions
of iron (II) ions