
Chemistry, 15.11.2019 19:31 makayyafreeman
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4(g) + 10 cl2(g) → 4pcl5(s) δh°rxn = ? given: pcl5(s) → pcl3(g) + cl2(g) δh°rxn= +157 kj p4(g) + 6 cl2(g) → 4 pcl3(g) δh°rxn = -1207 kj

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 01:30, jarteria0
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:00, sakria2002
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4...
Questions in other subjects:


Mathematics, 13.11.2020 22:50


Mathematics, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50


Geography, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

Mathematics, 13.11.2020 22:50

Spanish, 13.11.2020 22:50


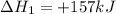 (1)
(1)
 (2)
(2)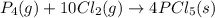
 (3)
(3)
 (4)
(4)


